Author Information1Cho, M.2; Herdrich, K.3; Jung, I.4; Liu, J.5; Wang, J.4; Yang, J.6
(Editor: Li, M.H.7)
1 All authors are listed in alphabetical order.
2 Episcopal High School, VA, 3 Troy High School, CA, 4 Flintridge Preparatory School, CA, 5 Mission San Jose High School, CA, 6 The Lawrenceville School, NJ
7 George Mason University, VA
Background: The gut microbiota, comprising the entire population of microorganisms that colonize the colon, has evolved over time to establish a symbiotic relationship with the human body, yielding mutual benefits [1]. This microbiome plays a pivotal role in critical bodily functions, including biosynthesis, short-chain fatty acid (SCFA) production, gut regulation, and immune system function. Under normal circumstances, these gut bacteria are referred to as indigenous microbiota, performing their customary functions; however, in the presence of disturbances, detrimental or opportunistic bacteria emerge, known as pathobionts [2]. Imbalances within the gut microbiota have been associated with various diseases, including obesity, metabolic disorders, inflammatory bowel diseases, colorectal cancer, allergies, and autoimmune disorders [1]. Of particular concern is the impact of immunosuppressants and antibiotics administered after kidney transplant surgeries, as they disrupt the patients’ gut microbiome, alter indigenous microbiota, and promote the proliferation of pathobionts, resulting in a condition known as dysbiosis. Dysbiosis, in turn, may facilitate the development of acute kidney injury (AKI) by modifying SCFA composition and generating elevated levels of toxins. Both AKI and pathobionts can contribute to the progression of atherosclerosis, cardiovascular diseases, inflammation, and chronic kidney disease (CKD). If left untreated, CKD can escalate to infections and even rejection of the newly transplanted kidney by the recipient’s immune system [2].
Objective: This project aims to 1) examine the degree of change occurred in the abundance and diversity of the gut microbiome, before and after kidney transplantation, and 2) identify treatments that support the microbiome in returning to its healthy, stable state.
Methods: The project dataset comprises data from 10 kidney transplant patients at the University of Toledo Medical Center, provided by our GMU faculty advisors. This dataset includes information on the gut microbiome species before and after transplantation for each patient. To analyze the patient data, we employed a t-test to identify microbiota species that exhibited significant changes in absolute abundance before and after transplantation. Furthermore, we utilized Pearson correlation analysis to identify microbiota species that displayed significant interactions before and after transplantation. Finally, we demonstrated that species with both significant changes in absolute abundance and significant interactions before and after transplantation. All statistical analyses were conducted using the R program, provided by our GMU faculty advisors.
Results: Out of the 141 microbiota species, only 5 exhibited significant changes in their absolute abundance, pre and post kidney transplantation. Phocaeicola contributes to colon health by breaking down complex heteropolysaccharides to SCFA [3]. Clostridium and Hungatella are harmful species that can cause diarrhea and inflammation in the gut [4]. Hungatella is associated with severe diseases such as septicemia (Juárez et al., 2021). Members of the Firmicutes species produce butyrate, an anti-inflammatory agent [5]. A decrease of this species suggests a decline in gut health and microbiome diversity. Streptococcus thermophilus is an important modulator of uremic toxins in the gut of patients diagnosed with chronic kidney disease [6]. In addition, our data analysis identified 78 species pairs with significant correlations. Of the 78 pairs, only 9 pairs (Hungatella and Clostridium) are shown here because they exhibited high correlations AND significant changes in absolute abundance pre- and post-transplantation.
Table 1. Species with Significant Change in Abundance
| Genus | Mean Difference | P-value |
| Phocaeicola | 15219.09 | 0.035 |
| Hungatella | 1134.68 | 0.062 |
| Clostridium | 8279.27 | 0.09 |
| Firmicutes_u_g | -13113.33 | 0.033 |
| Streptococcus | 2704.62 | 0.095 |
Table 2. Species with Significant Interactions
| Genus 1 | Genus 2 | Pearson Correlation Coefficient | P-value |
| Hungatella | Intestinimonas | -0.79 | 0.007 |
| Hungatella | Coprobacillus | 0.85 | 0.002 |
| Hungatella | Erysipelatoclostridium | 0.97 | <0.001 |
| Clostridium | Faecalibacterium | 0.77 | 0.009 |
| Clostridium | Oscillibacter | 0.81 | 0.004 |
| Clostridium | Flavonifractor | 0.83 | 0.003 |
| Clostridium | Dysosmobacter | 0.8 | 0.005 |
| Clostridium | Clostridia_u_g | 0.78 | 0.008 |
| Clostridium | Lachnospiraceae_u_g | 0.79 | 0.007 |
Figure 1: Microbial Network Change After Kidney Transplant
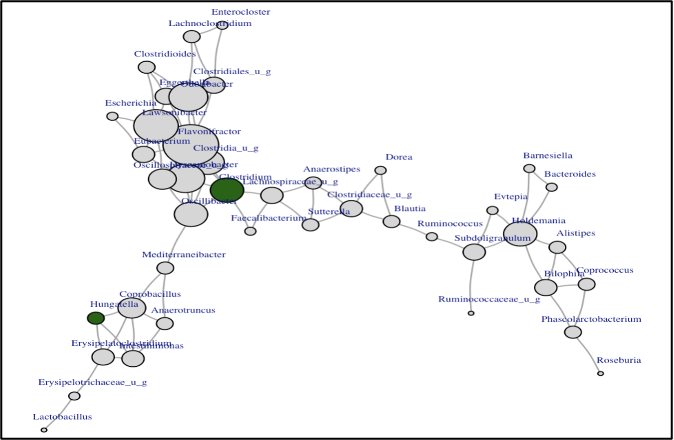
Conclusions: Through patient data analysis and literature research, this project identified the most impactful bacteria that could play a key role in restoration of patients’ gut microbiome diversity, richness, and overall health.
References
- Jandhyala, S.M., Talukdar, R., Subramanyam, C., Vuyyuru, H., Sasikala, M. and Reddy, D.N., 2015. Role of the normal gut microbiota. World journal of gastroenterology: WJG, 21(29), p.8787.
- Salvadori, M., 2021. The Microbiota and Kidney Transplantation: Influence on the Graft. Urology, 9, pp.95-105.
- Lück, R. and Deppenmeier, U., 2022. Genetic tools for the redirection of the central carbon flow towards the production of lactate in the human gut bacterium Phocaeicola (Bacteroides) vulgatus. Applied Microbiology and Biotechnology, 106(3), pp.1211-1225.
- Mayo Clinic. (2021, August 27). C. difficile infection. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/c-difficile/symptoms-causes/syc-20351691.
- Edermaniger, L. (2021, June 21). Firmicutes bacteria: What are they and why are they important? Atlas Biomed blog | Take control of your health with no-nonsense news on lifestyle,
- Gut microbes and genetics. https://atlasbiomed.com/blog/guide-to-firmicutes/
- Vitetta, L., Chen, J. and Clarke, S., 2019. The vermiform appendix: an immunological organ sustaining a microbiome inoculum. Clinical Science, 133(1), pp.1-8.
